
Iain Dale 10am - 12pm
28 May 2021, 16:14
The recommendation follows similar decisions by regulators in Canada and the US last month.
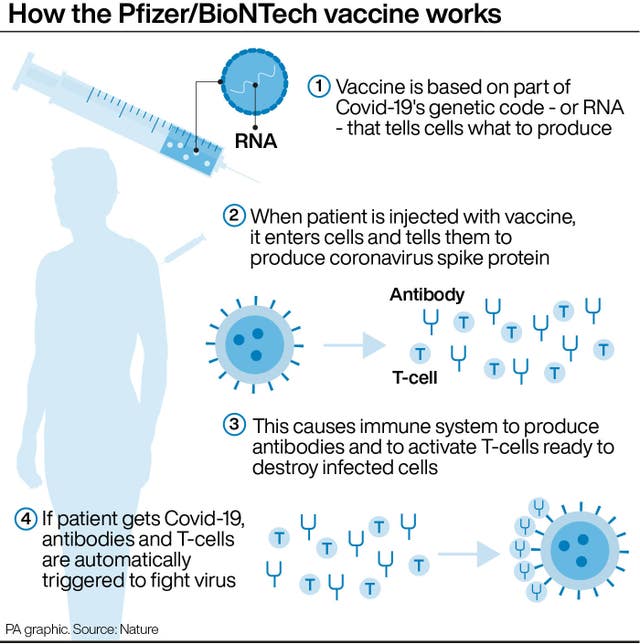
The European Medicines Agency has recommended the use of the vaccine made by Pfizer and BioNTech be expanded to children aged 12 to 15, offering younger and less at-risk populations across the continent access to a Covid-19 jab for the first time during the pandemic.
Marco Cavaleri, who heads the EMA, said the European Union regulator had received the necessary data to authorise the vaccine for younger teenagers and found it to be highly effective against Covid-19.
The decision needs to be rubber-stamped by the European Commission and individual national regulators, he said.
The recommendation follows similar decisions by regulators in Canada and the US last month, as rich countries slowly approach their vaccination targets for adults and look to immunise as many people as possible.
The Pfizer-BioNTech COVID-19 vaccine was the first one granted authorization across the 27-nation EU when it was licensed for use in anyone 16 and over in December.
The EMA’s recommendation was based on a study in more than 2,000 adolescents in the US that showed the vaccine was safe and effective.
Researchers will continue to monitor the jab’s long-term protection and safety in the children for another two years.
Most Covid-19 vaccines worldwide have been authorised for adults, who are at higher risk of severe disease and death from the coronavirus, but vaccinating children of all ages could be critical to stopping outbreaks, since some research has shown older children may play a role in spreading the virus even though they do not typically fall seriously ill.
In the US, children represent about 14% of the country’s coronavirus cases and at least 316 have died, according to the American Academy of Paediatrics. Doctors have also identified a rare inflammatory syndrome in a very small proportion of children suffering with Covid-19.
Immunising children against Covid-19 might also give authorities more confidence to reopen schools, as getting children to wear masks and social distance has been challenging.
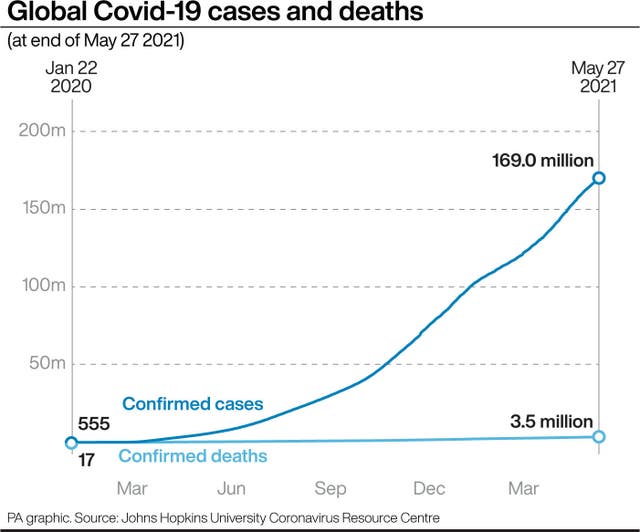
But the World Health Organisation has criticized rich countries for moving on to vaccinate their younger and less at-risk populations, saying that the extremely limited number of Covid-19 vaccines should be shared with poor countries so they can protect their health workers and those most vulnerable.
“I understand why some countries want to vaccinate their children and adolescents, but right now I urge them to reconsider and to instead donate vaccines to Covax,” WHO chief Tedros Adhanom Ghebreyesus said earlier this month, referring to the UN-backed initiative to distribute vaccines.
Of more than a billion Covid-19 jab administered globally, fewer than 2% have gone to poor countries.
Other vaccine makers are studying whether their jabs are safe and protective in children.
Earlier this week, Moderna said its jab strongly protects children as young as 12. It said it would submit a request for emergency use authorisation to the US Food and Drug Administration next month.
Another US company, Novavax, has a vaccine in late-stage development and has started study in 12 to 17-year-olds.
Moderna and Pfizer-BioNTech have been testing their vaccines in children from the age of 11 down to six months. They get a lower dose than teenagers and adults.
China’s Sinovac has also submitted early data to the country’s regulators, hoping to prove its vaccine is safe in children as young as three.