
Clive Bull 1am - 4am
7 May 2021, 17:04
The decision by a technical advisory group opens the possibility that the jab could be included in the UN-backed Covax programme.
The World Health Organisation has given authorisation for emergency use of a Covid-19 vaccine manufactured by China’s Sinopharm, potentially paving the way for millions of doses to reach needy countries through a UN-backed programme.
The decision by a WHO technical advisory group opens the possibility that the Sinopharm vaccine could be included in the UN-backed Covax programme in coming weeks or months, and distributed through Unicef and the WHO’s regional office in the Americas.
Sinopharm has released little data publicly, aside from efficacy numbers for its two jabs – one developed by the Beijing Institute of Biological Products and the other by the Wuhan Institute of Biological Products.
The Beijing jab is one that was considered by the WHO for the emergency use listing.
"This afternoon, WHO gave Emergency Use Listing to Sinopharm Beijing’s #COVID19 vaccine, making it the sixth vaccine to receive WHO validation for safety, efficacy and quality."- @DrTedros
— World Health Organization (WHO) (@WHO) May 7, 2021
“This afternoon, WHO gave emergency use listing to sign off on Beijing’s Covid-19 vaccine, making it the sixth vaccine to receive WHO validation for safety, efficacy and quality,” director-general Tedros Adhahom Ghebreysus said.
The Sinopharm vaccine will join ones made by Pfizer-BioNTech, Johnson & Johnson, Moderna, AstraZeneca, and a version of the AstraZeneca vaccine made by the Serum Institute of India, in receiving the coveted authorisation from the UN health agency.
“This expands the list of vaccines that Covax can buy and gives countries confidence to expedite their own regulatory approval and to import and administer a vaccine,” Mr Tedros said at a Geneva news conference.
A separate group advising the WHO on vaccines had previously said it was “very confident” the Sinopharm vaccine protects people aged 18-59, but had a “low level of confidence” in efficacy for people aged 60 and over.
Sinopharm has not published late-stage test results in scientific journals, so the WHO requested a breakdown of data, which comes mostly from the United Arab Emirates.
A summary posted online by the WHO suggests the vaccine is about 78% effective, with the caveat that all but a few hundred of the study volunteers were younger than 60.
Gavi, the Vaccine Alliance, which co-runs Covax, welcomed the announcement, saying: “This means the world has yet another safe and effective tool in the fight against this pandemic.”
Help fund vaccine doses by donating to https://t.co/Wh1muHGDRy or help to distribute them at https://t.co/fnSP6jx0zt
Let's get vaccines to ALL parts of the world! 🌎🌍🌏
Special thanks to @UNICEF and @gavi for supporting such an important message sponsored by @wellcometrust .
— Lily Hevesh (@Hevesh5) May 7, 2021
The public-private partnership said it was in discussions with several manufacturers, including Sinopharm, “to expand and diversify the portfolio further and secure access to additional doses” for countries in the Covax programme.
Covax aims to send vaccines for free to 92 lower-income countries and to help another 99 countries and territories procure them.
The programme, which has already distributed more than 54 million doses of Covid-19 vaccines but faces limited supplies from Western countries and India, has been working hard to strike deals as part of its goal to procure two billion doses by the end of the year.
Suerie Moon, co-director of the Global Health Programme at Geneva’s Graduate Institute, said the WHO decision on the Sinopharm vaccine and other Chinese jabs will “carry a lot of weight” because of limited information publicly available about them.
“The decision is also sure to be scrutinised all around for any whiff of political bias, and no doubt the committee members were very well aware of this,” she said, noting that the decision could also be a boon for developing countries in need of coronavirus vaccines.
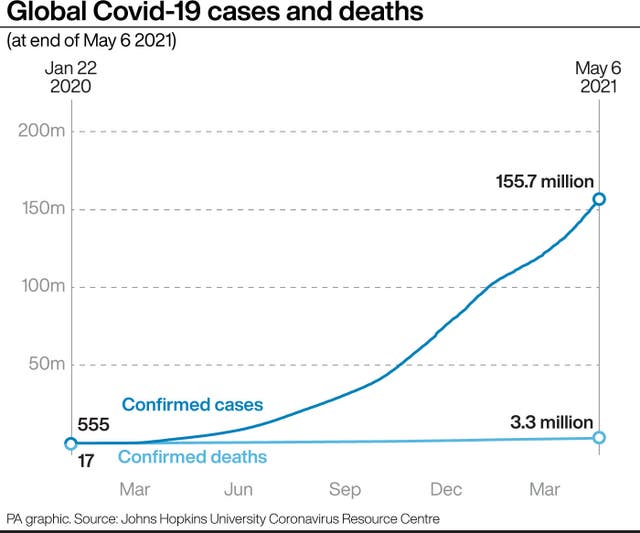
The WHO decision on Sinopharm, months in the making, was particularly complex because the vaccine has not faced the high-level scrutiny of a rigorous medicines regulator like those in Europe and the US.
The WHO panel relied frequently on those Western agencies’ findings when it came to vaccines it has approved for emergency use.
Hundreds of millions of Chinese vaccines have already been delivered to dozens of countries through bilateral deals as many scrambled to secure supplies after rich countries reserved the vast majority of supplies from Western pharmaceutical makers.
While China has five jabs in use, the majority of its exports come from Sinopharm and Sinovac. A decision on Sinovac is expected next week, the WHO said.
Sinopharm said last month that more than 100 million doses of its two vaccines have been used across the world.
Sinovac has shared more data. Last month, a study by a team of scientists in Brazil confirmed a previously reported efficacy rate of over 50%, and a real-world study in Chile found an efficacy rate of 67%.