
Ian Payne 4am - 7am
31 March 2021, 12:04

Most vaccines being rolled out worldwide are for adults, who are at higher risk from coronavirus.
Pfizer has announced its Covid-19 vaccine is safe and strongly protective among children as young as 12, a step towards possibly beginning jabs in this age group before they head back to school in the autumn.
Most Covid-19 vaccines being rolled out worldwide are for adults, who are at higher risk from the coronavirus.
Pfizer’s vaccine is authorised for people aged 16 and older, but vaccinating children of all ages will be critical to stopping the pandemic — and helping schools return to normal after months of disruption.
In a study of 2,260 US volunteers aged 12 to 15, preliminary data showed no cases of Covid-19 among fully vaccinated adolescents, compared with 18 among those given dummy shots, Pfizer reported.
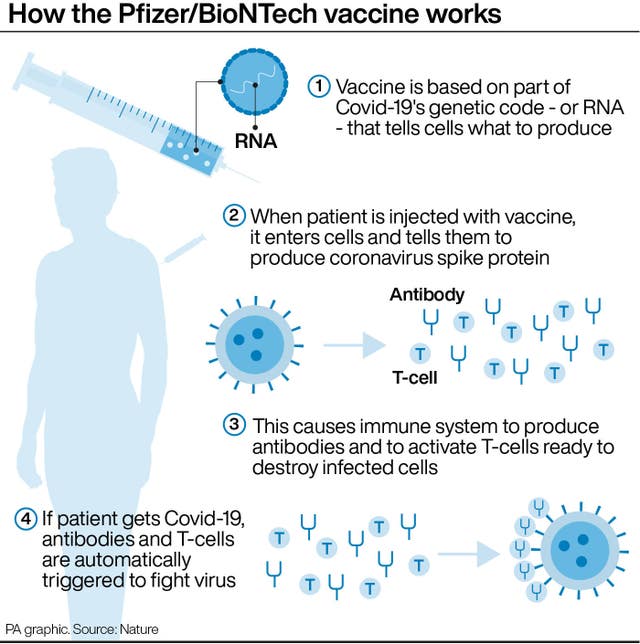
It is a small study that has not yet been published, so another important piece of evidence is how well the jabs boosted the children’s immune systems. Researchers reported high levels of virus-fighting antibodies, higher than in studies of young adults.
Children had side effects similar to young adults, the company said, mainly pain, fever, chills and fatigue, particularly after the second dose.
The study will continue to track participants for two years for more information about long-term protection and safety.
Pfizer and its German partner BioNTech in coming weeks plan to ask the US Food and Drug Administration and European regulators to allow emergency use of the vaccine starting at the age of 12.
“We share the urgency to expand the use of our vaccine,” Pfizer chief executive Albert Bourla said in a statement. He expressed “the hope of starting to vaccinate this age group before the start of the next school year” in the US.
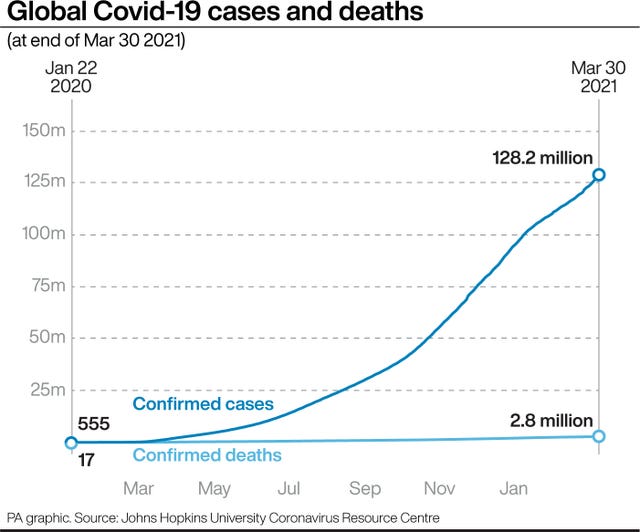
Pfizer is not the only company seeking to lower the age limit for its vaccine. Results also are expected soon from a US study of Moderna’s vaccine in 12 to 17-year-olds.
But in a sign that the findings were promising, the FDA has already allowed both companies to begin US studies in children aged 11 and younger, working their way to as young as six months.
AstraZeneca last month began a study of its vaccine among six to 17-year-olds in Britain, Johnson & Johnson is planning its own paediatric studies, and in China, Sinovac recently announced it had submitted preliminary data to Chinese regulators showing its vaccine is safe in children as young as three.
While most Covid-19 vaccines being used globally were first tested in tens of thousands of adults, paediatric studies will not need to be nearly as large. Scientists have safety information from those studies and from subsequent vaccinations in millions more adults.