
Nick Ferrari 7am - 10am
13 April 2021, 17:24
Hundreds of thousands of doses of the vaccine were due to be shipped to Europe in the coming weeks.
Johnson & Johnson has said it is delaying the rollout of its coronavirus vaccine in Europe amid a US probe into reports of rare blood clots in some recipients.
The announcement came after regulators in the United States said they were recommending a “pause” in administration of the single-dose vaccine to investigate reports of potentially dangerous blood clots.
“We have been reviewing these cases with European health authorities,” Johnson & Johnson said.
“We have made the decision to proactively delay the rollout of our vaccine in Europe.”

The delay is a further blow to vaccination drives in the European Union, which have been plagued by supply shortages, logistical problems and concerns over unusual blood clots in a small number of people who received the AstraZeneca vaccine.
The blood clot reports prompted several countries in the 27-nation bloc to limit the AstraZeneca vaccine to older age groups, which are more at risk from serious illness when infected with Covid-19.
The Johnson & Johnson and AstraZeneca vaccines are made with the same technology.
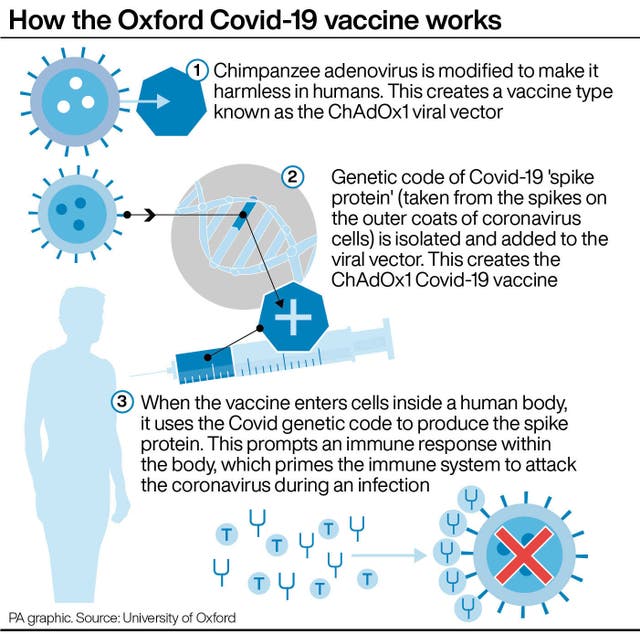
Many leading Covid-19 vaccines train the body to recognise the spike protein that coats the outer surface of the coronavirus.
But the J&J and AstraZeneca vaccines use a cold virus, called an adenovirus, to carry the spike gene into the body.
Johnson & Johnson uses a human adenovirus to create its vaccine, while AstraZeneca uses a chimpanzee version.
The European Medicines Agency, the EU’s equivalent to the US Food and Drug Administration (FDA), said last Friday that it was reviewing cases reported in the United States of blood clotting in people who had received the J&J vaccine, which was developed by the company’s Janssen subsidiary.
In a joint statement on Tuesday, the Centres for Disease Control and Prevention (CDC) and the FDA said they were investigating unusual clots that occurred six to 13 days after vaccination.
The clots occurred in veins that drain blood from the brain and occurred together with low platelets.
All six cases were in women between the ages of 18 and 48; there was one death.
The Amsterdam-based EMA said following the US announcement that it “is currently not clear whether there is a causal association between vaccination with Covid-19 Vaccine Janssen and these conditions”.
The European agency’s safety committee “is investigating these cases and will decide whether regulatory action may be necessary”, the EMA said.
The EU ordered 200 million doses of the Johnson & Johnson vaccine in 2021.
Britain ordered 30 million doses of the J&J vaccine, though UK regulators have not yet approved its use.
France had expected to receive 200,000 doses of the vaccine this week and was planning to start administering them next week to people aged 55 and over.
In total, the country had planned to receive about eight million doses of the J&J vaccine by the end of June.

In February, South Africa began vaccinating its health workers with the J&J vaccine in a research trial after abandoning plans to use the AstraZeneca shot when a preliminary study suggested the AstraZeneca vaccine was only minimally effective against the variant of Covid-19 that first arose in the country.
South Africa planned to test the J&J shot in 500,000 health workers as part of the research, and as of Monday night, it had vaccinated 289,787.
Last month, the African Union announced it had signed a deal to buy up to 400 million doses of the J&J vaccine.
Johnson & Johnson also has a preliminary agreement to supply up to 500 million doses through until 2022 to the UN-backed Covax initiative, an effort to provide Covid-19 vaccines to the world’s poor.
The World Health Organisation (WHO) last month gave the green light to the J&J vaccine, saying its formulation “should facilitate vaccination logistics in all countries”.
Any concerns about the J&J vaccine could be another unwelcome complication for Covax and for the billions of people in developing countries depending on the programme to get immunised.
Covax was recently hit by supply issues after its biggest supplier, the Serum Institute of India, announced it would delay exports of the AstraZeneca vaccine for several months due to a surge of cases in the subcontinent.
European regulators on March 11 endorsed the Johnson & Johnson vaccine as the fourth authorised for use in the EU, saying the data on the product indicated it met the bloc’s criteria for efficacy, safety and quality, but the first supplies were only now arriving.
Italy expected to receive its first Johnson & Johnson deliveries this week.
The Lazio region surrounding Rome planned to give the prison population the single jab, while the northern Veneto region, which includes Venice, planned to use it for housebound adults over 80.
“I am watching today’s news with concern, as a humanitarian actor. I am also watching with satisfaction, because the regulatory system is working,” Francesco Rocca, president of the International Federation of the Red Cross, told foreign reporters in Italy.
“I imagine there will be repercussions, as we are waiting for millions of doses. But this means the controls are working. If we need to be prudent, we need to be prudent.”
Officials in Germany, which was due to receive 232,800 doses of the vaccine this week and 10.1 million doses by the end of June, said earlier on Tuesday that there were no immediate plans to change the schedule.
“I don’t currently have the date from which Johnson & Johnson will be administered,” Health Ministry spokesman Hanno Kautz told reporters in Berlin.
“But in principle, we naturally always take such warnings in an international context seriously and investigate them.”

Spain is expecting to receive 300,000 doses of J&J on Wednesday, the first delivery of the jab produced by Johnson & Johnson.
Asked about the pause on Johnson & Johnson shots in the United States, Spanish Prime Minister Pedro Sanchez said the benefits of taking the vaccine remain greater than the risks but that he would have to learn more about the situation.
“We have to all be aware that all the vaccines being administered to the Spanish population, and the European, as well as American populations, are safe,” Mr Sanchez said.