
Ali Miraj 12pm - 3pm
20 September 2021, 12:14
The vaccine maker said it plans to seek authorisation for this age group in the US, the UK and Europe.
Pfizer has said its Covid-19 vaccine works for children aged five to 11 and it will seek US authorization for the age group soon.
The vaccine made by Pfizer and its German partner BioNTech is already available for anyone 12 and older, but with children now back in school and the extra-contagious Delta variant causing a huge jump in paediatric infections, many parents are awaiting vaccinations for their younger children.
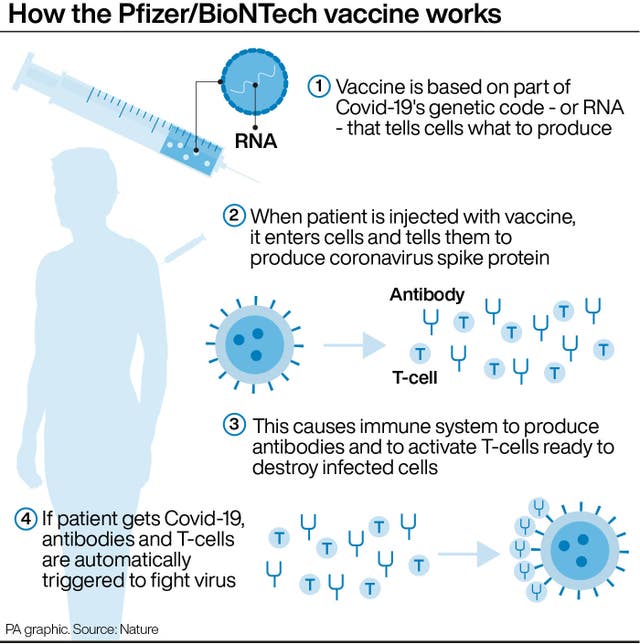
For primary school-aged children, Pfizer tested a much lower dose – a third of the amount in each jab given now. After the second dose, children aged five to 11 developed coronavirus-fighting antibody levels just as strong as teenagers and young adults, Dr Bill Gruber, a Pfizer senior vice president, told the Associated Press.
The dosage also proved safe, with similar or fewer temporary side effects – such as sore arms, fever or achiness – that teenagers experience, he said.
“I think we really hit the sweet spot,” said Dr Gruber, who is a paediatrician.
He said the companies aim to apply to the US Food and Drug Administration by the end of the month for emergency use in this age group, followed shortly afterwards with applications to European and British regulators.
Earlier this month, FDA chief Dr Peter Marks told the AP that once Pfizer turns over its study results, his agency will evaluate the data “hopefully in a matter of weeks” to decide if the jabs are safe and effective enough for younger children.
Many Western countries have vaccinated no younger than age 12, but Cuba last week began immunising children as young as two with its homegrown vaccines and Chinese regulators have cleared two of its brands down to the age of three.
“I feel a great sense of urgency” in making the vaccine available to children under 12, Dr Gruber said. “There’s pent-up demand for parents to be able to have their children returned to a normal life.”
Pfizer said it studied the lower dose in 2,268 nursery and primary school-aged children.
The FDA required what is called an immune “bridging” study: evidence that the younger children developed antibody levels already proven to be protective in teenagers and adults.
That is what Pfizer reported on Monday in a press release, not a scientific publication. The study is ongoing, and there have not yet been enough Covid-19 cases to compare rates between the vaccinated and those given a placebo — something that might offer additional evidence.
A second US vaccine maker, Moderna, is also studying its jabs in primary school children. Pfizer and Moderna are studying even younger children as well, down to six months old, and results are expected later in the year.