
Clive Bull 1am - 4am
11 May 2021, 06:43
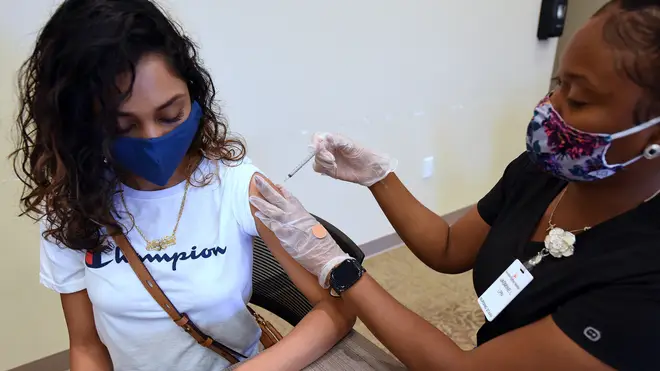
The US has authorised the Pfizer-BioNtech Covid-19 vaccine for children aged 12 to 15, it has been announced.
It is hoped the jab rollout will provide a way to protect the nation's adolescents before they head back to school in autumn.
Shots could begin as soon as Thursday after a federal vaccine advisory committee issues recommendations for using the two-dose vaccine in 12- to 15-year-olds, with an announcement expected on Wednesday.
Most Covid-19 vaccines worldwide have been authorised for adults.
Read more: PM confirms Covid restrictions to be lifted on 17 May - all you need to know
Pfizer's vaccine is being used in multiple countries for teens as young as 16, with Canada recently becoming the first to expand use to 12 and up.
Parents, school administrators and public health officials elsewhere have eagerly awaited approval for the shot to be made available to more kids.

"This is a watershed moment in our ability to fight back the Covid-19 pandemic," Pfizer senior vice president doctor Bill Gruber, told The Associated Press.
The Food and Drug Administration (FDA) declared that the Pfizer vaccine is safe and offers strong protection for younger teens based on testing of more than 2,000 US volunteers ages 12 to 15.
The agency noted there were no cases of Covid-19 among fully vaccinated adolescents compared with 16 among kids given dummy shots.
Read more: Covid alert level lowered from four to three ahead of May 17 lockdown easing
More intriguing, researchers found the kids developed higher levels of virus-fighting antibodies than earlier studies measured in young adults.
The younger teens received the same vaccine dosage as adults and had the same side effects, mostly sore arms and flu-like fever, chills or aches that signal a revved-up immune system, especially after the second dose.
Govt scientist's instant reaction to PM's lockdown easing
Pfizer's testing in adolescents "met our rigorous standards", FDA vaccine chief doctor Peter Marks said.
"Having a vaccine authorised for a younger population is a critical step in continuing to lessen the immense public health burden caused by the Covid-19 pandemic," he added.
Pfizer and its German partner BioNTech recently requested similar authorisation in the European Union, with other countries to follow.
The latest news is welcome for US families struggling to decide what activities are safe to resume when the youngest family members remain unvaccinated.