
Iain Dale 10am - 1pm
20 December 2019, 08:07
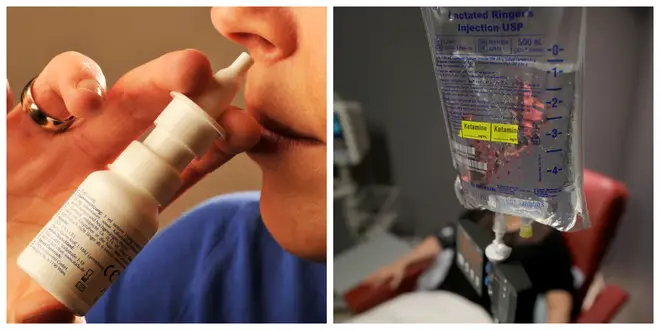
An anti-depressant derived from ketamine has been licensed for use in Britain after being approved by the European Commission, according to the US manufacturer of the drug.
The nasal spray Esketamine is used to treat severe depression in patients who have not responded well to traditional anti-depressants.
The fast-acting treatment spray is already sold in the US by manufacturer Janssen under the brand name Spravato.
It could now be available on the NHS within months after Janssen announced that the European Commission had approved the drug.
Read: Mum's horror as son, 10, bites into banana which had needle inside
The National Institute for Health and Care Excellence (Nice) is expected to rule by June next year whether the drug will be made available in the UK.
However, experts say that a cost negotiation will have to take place before it is rolled out on the NHS.
The Medicines and Healthcare products Regulatory Agency (MHRA) will also decide whether to impose any additional safety measures on the drug.
The drug works faster than traditional anti-depressants, with patients shown to benefit within hours, rather than weeks or months to start having an effect.
Dr James Stone, clinical senior lecturer in psychiatry at King's College London, has worked on several studies on the drug's use as an alternative to traditional anti-depressants.
He said: "We have known for some time that ketamine has anti-depressant effects in people who have treatment resistant depression (TRD), who have not responded to other treatments.
"Ketamine is unique in that the effect starts within one hour compared to other medicines that can take two to four weeks to have an effect.
"Up to now it has been given as a slow injection into the veins in the arm but has been given as an unlicensed medicine.
"Esketamine is a form of ketamine available as a nasal spray and will be the first form of this medicine to be licensed in the UK.
"As such it represents a potential breakthrough treatment for people with depression who have not responded to any other treatments.
"It is likely that it will only be given in a day patient setting, given to patients who have to attend the hospital each time.
"The downsides to it are that maintaining response will require twice weekly visits to hospital, for the first few weeks of treatment. It will then require weekly visits for several months.
"Although there have not been some of the problems associated with heavy use in ketamine addicts, it is likely that problems around ketamine use could emerge - especially if use and prescribing is not closely monitored."
He added that physical side effects, such as changes in perception of reality, changes in blood pressure and nausea, are common for about an hour after treatment.
"Although it has not been closely studied, there is a risk that people may start taking ketamine illegally and put themselves at risk of addiction," he said.
"Lastly, it is likely to be expensive and this may make the treatment impractical or impossible to access in many NHS trusts."