
Ian Payne 4am - 7am
24 August 2020, 05:55
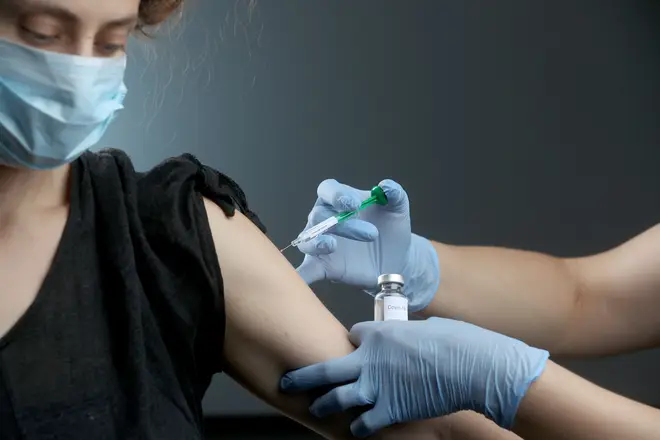
The White House is considering fast-tracking an experimental coronavirus vaccine from the UK for use in America ahead of the presidential election, it has been reported.
President Donald Trump's administration is looking at the potential vaccine being developed by Oxford University and AstraZeneca, according to a report by the Financial Times.
One option open to the President could involve the US Food And Drug Administration (FDA) authorising it for emergency use in October, a month before Mr Trump faces Democratic rival Joe Biden for the presidency, the paper reports.
Human trials of the coronavirus vaccine candidate being developed at the University of Oxford suggest it is safe and induces an immune response to Covid-19.
Early results indicate the jab could provide double protection - generating an immune response which stimulates the body to produce both an antibody and T-cell response.
But what is the US president considering, what does the vaccine do and how does it work?
A report in the Financial Times said he was considering bypassing normal US regulatory standards to fast-track the coronavirus vaccine being developed at the University of Oxford for use in America.
The paper said one option being explored would involve the US Food and Drug Administration awarding "emergency use authorisation" to a vaccine in October.

‘This is for the greater good’: LBC speaks to woman taking part in covid-19 vaccine trial
The Financial Times reported that the US government's scientific agencies have said a vaccine would need to be studied in 30,000 people to pass the threshold for authorisation.
The Oxford vaccine is recruiting around 12,000 people for the next phase of human trials.

William congratulates Oxford vaccine trial team
The vaccine - called ChAdOx1 nCoV-19 - uses a weakened version of a common cold virus (adenovirus) which causes infections in chimpanzees.
It has been genetically changed so it is impossible for it to grow in humans.
It is hoped the vaccine will make the body recognise and develop an immune response to the spike protein - recognisable in images of the virus - that will help stop Covid-19 from entering human cells and therefore prevent infection.
The results of the clinical trials, published in The Lancet last month, indicate that the vaccine candidate has triggered two responses in the immune system.
The first is that it stimulates the immune system to produce antibodies - proteins produced by the blood in response to antigens which are harmful substances that come from outside the body, such as from viruses or bacteria - and that it also causes the body to produce T-cells.
If the non-specific immune cells which respond to any invader instantly cannot tackle it, the T-cells come into play.
These cells attack the virus directly.
With questions remaining about the duration of the antibody response to Covid-19, research suggests T-cells have a more important role in offering protection against the disease.
Sarah Gilbert, professor of vaccinology at Oxford University, explained that T-cells recognise and kill cells that have been taken over by a virus and been turned into little "virus factories".
She added: "The two systems working together are completely complementary, first of all stopping infection coming in, and if (the virus) does get past the antibodies, (T-cells) destroy the cells that the virus has taken over."
It is not yet known whether the Oxford vaccine candidate provides long-term immunity.

Lead scientist in Imperial vaccine trials briefs ins and outs of trial
No.
Based on their results, researchers say further clinical studies should be done with this vaccine.
The current results focus on the immune response measured in the laboratory, and further testing is needed to confirm whether the vaccine effectively protects against infection.
Thousands of participants are already enrolled in the UK, with enrolment of a further 10,000 people planned as researchers test the ChAdOx1 nCoV-19 vaccine.
Trials are also taking place in South Africa and Brazil and it is hoped an effective vaccine could be ready later this year.
This trial aims to assess how well people across a broad range of ages could be protected from Covid-19.
Production of the vaccine has already been scaled up ahead of the trial to prepare as early as possible for potential future deployment.
The UK Government has signed deals for 90 million doses of promising Covid-19 vaccines, with more in the pipeline.
The latest deal is for vaccines being developed by an alliance between the pharmaceutical giants BioNtech and Pfizer as well as the firm Valneva.
This is in addition to 100 million doses of a vaccine being developed by Oxford University with AstraZeneca.