
Richard Spurr 1am - 4am
4 June 2021, 11:19 | Updated: 4 June 2021, 22:52

Pfizer-BioNTech Covid-19 vaccine approved for use in 12 to 15-year-olds
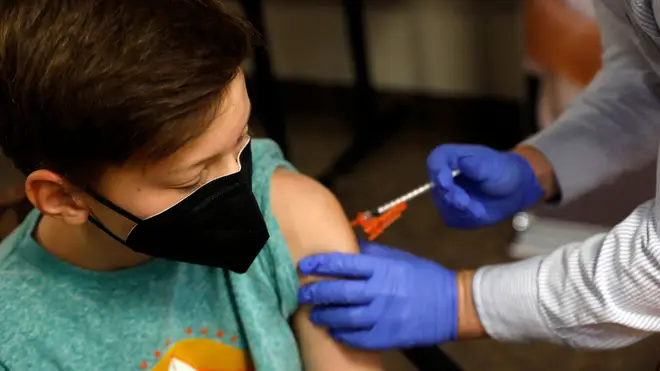
The UK's medicines regulator has approved the use of the Pfizer vaccine in 12 to 15-year-olds.
The MHRA has approved an extension to the current UK approval of the Pfizer/BioNTech COVID-19 vaccine that allows its use in 12-15 year olds, although at present there are no firm plans to routinely vaccinate under 18s.
The vaccine, which was already approved for use in over 16s, has been found to be safe for 12-15 year olds after no new side affects were identified.
A Department of Health and Social Care spokesperson said: “Following a robust review of the evidence, the MHRA has concluded that the Pfizer/BioNTech COVID-19 vaccine meets the high standards of safety, effectiveness and quality required and has authorised its use for young people aged 12 to 15.
Read more: Pfizer jab recipients have lower antibodies against Indian variant, study suggests
Read more: Toppled Edward Colston statue goes on display in Bristol
“The government has asked the independent experts at the Joint Committee on Vaccination and Immunisation (JCVI) to advise whether routine vaccination should be offered to younger people aged 12 to 17.
“We will be guided by the expert advisors and will update in due course.”
At present, the JCVI have only laid out plans to vaccinate over 18s.
However, outbreaks in schools are thought to be contributing to surges in the Delta variant, so the announcement by the MHRA may prompt children to be included at some stage of the vaccine rollout.
Some countries, such as Germany and France, have already announced plans to begin vaccinating children.
Epidemiologist hits out at government rules on travel
The side effects reported in 12-15 year olds were similar to those reported in young adults.
They tended to be mild to moderate, and related to expected symptoms such as having a sore arm or feeling tired.
Dr Julie Raine, MHRA Chief Executive, said that 12-15 year olds given the vaccine will continue to be monitored.
“We have in place a comprehensive safety surveillance strategy for monitoring the safety of all UK-approved COVID-19 vaccines and this surveillance will include the 12- to 15-year age group," she said.
“No extension to an authorisation would be approved unless the expected standards of safety, quality and effectiveness have been met."