
Shelagh Fogarty 1pm - 4pm
11 February 2021, 15:05 | Updated: 11 February 2021, 17:03
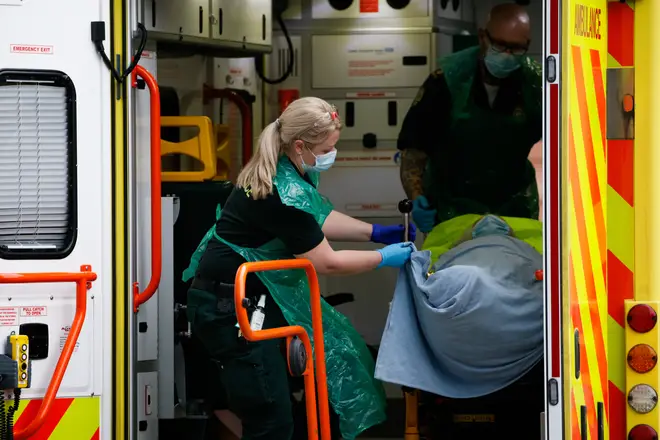
Thousands of Covid-19 patients are to receive a "life-saving" arthritis drug that has been found to substantially cut the risk of death and need for a ventilator.
Tocilizumab, usually used to treat rheumatoid arthritis, has been approved for use to treat patients suffering from the virus in NHS hospitals.
The drug reduced the relative risk of death by 14% and reduced the time spent in hospital by five days when used for patients on oxygen and in addition to the corticosteroid dexamethasone.
Read more: AstraZeneca looking to roll out jab for new Covid-19 variants by Autumn
Read more: SAGE scientist predicts UK should be 'more or less' free of Covid crisis by end of 2021
The rollout of the treatment could also significantly reduce pressures on hospitals over the coming weeks and months.
Scientists discovered the drug was effective during the RECOVERY clinical trial, funded by the UK government through the National Institute for Health Research (NIHR) and UK Research and Innovation (UKRI).

Virologist explains why Kent variant is 'on course to sweep the world'
Last month, the international clinical trial REMAP-CAP, also funded by the government, found that tocilizumab and sarilumab reduced the risk of death for patients when administered within 24 hours of entering intensive care.
Health secretary Matt Hancock said: “Today’s excellent news is further proof the UK is at the forefront of the global mission to find safe and effective treatments for this terrible virus.
Read more: One third of all Covid-19 patients in England's hospitals admitted in January 2021 alone
“I want to thank all those who have played a part in generating these tremendous results - from the British scientists and researchers behind the trial, to the thousands of patients who took part across the country.
“We are working quickly and closely with colleagues across the health system and sector to ensure every NHS patient who needs this treatment should be able to access it - reducing further pressures on the NHS and potentially saving thousands of lives.”
Dr Ray Sheridan, who is part of the trial at the Royal Devon & Exeter hospital, told LBC that mortality in the sickest intensive care patients could be reduced "by about half" when the drug is combined with steroids.
He said: "This next stage of the RECOVERY trial has given us an extra drug that works, not just on its own but also on top of the benefit that you see from steroids.
"You can combine these on top of tocilizumab, which is an anti-inflammatory drug normally use in rheumatoid arthritis we have readily available in our fridges in hospital wards or pharmacies.
"This drug controls something called interleukin six and it blocks those receptors and that's something controls infection and our immune system."
UK must not set a date for ending lockdown, GP tells LBC
He said that when combined with steroids, the drug can "save lives and shorten the stay" for many Covid-19 patients.
Commenting on the results of the trial, Dr Sheridan added: "Those results are really big numbers, so you only have to treat 25 patients on a ward with Covid, so treat 25 patients and one more person goes home to their family, so that's really great news for us."
Deputy chief medical officer professor Jonathan Van-Tam said the approval of the drug was "another important advance" in the fight against the virus.
Read more: Kent Covid-19 variant 'to become world's dominant strain'
He said: “The data published today mean many more patients in hospital with COVID-19 will have access to a proven treatment, speeding up their recovery and reducing the risk of mortality significantly.
“It’s because of the UK’s world-class clinical trials infrastructure, including NIHR infrastructure in NHS hospitals, and the generosity of UK patients to volunteer even though they are ill themselves, that trials like RECOVERY are able to deliver definitive evidence that will save lives, and I am hugely grateful to all those involved.”