
Ian Payne 4am - 7am
2 February 2021, 14:29
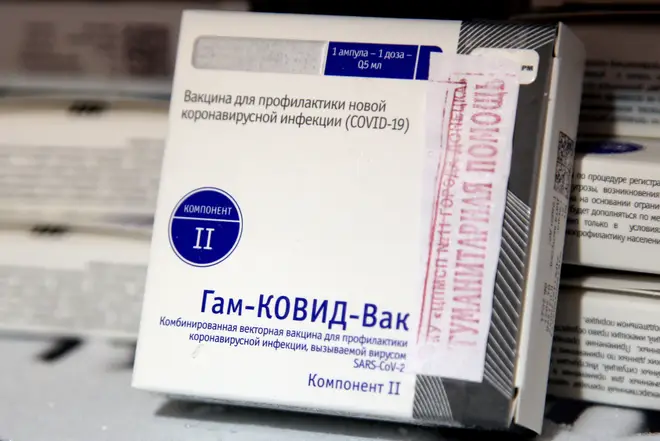
Russia's Covid-19 vaccine is 91.6% effective against symptoms, interim trial results suggest.
Data from the phase three trial of the vaccine - officially named Gam-COVID-Vac (Sputnik V) - suggests a two-dose regimen provides one of the highest efficacies so far.
No serious adverse effects were associated with vaccination and most which were reported were mild, including flu-like symptoms, pain at the injection site and weakness or low energy.
The preliminary findings, published in The Lancet, are based on analysis of data from nearly 20,000 participants - three quarters of whom received the jab and one quarter received a placebo.
Read more: Mutation of Kent Covid-19 variant 'could impact effectiveness of some vaccines'
The jab delivers adenovirus vectors - recombinant human adenovirus type 26 (rAd26-S) and recombinant human adenovirus type 5 (rAd5-S) - which have been modified to combat the SARS-CoV-2 spike protein.
Adenoviruses are also used in the production of the Oxford-AstraZeneca and Janssen drugs and are weakened so they cannot replicate in human cells and cause disease.

Matt Hancock says 9.2 million people in UK have been vaccinated
According to the researchers, using a different adenovirus vector for the booster vaccination may help create a more powerful immune response, compared with using the same vector twice.
They suggest this is because it minimises the risk of the immune system developing resistance to the initial jab.
Dr Inna V Dolzhikova, co-lead author, from the Gamaleya National Research Centre for Epidemiology and Microbiology in Russia, said: "Our interim analysis of the randomised, controlled, phase 3 trial of Gam-COVID-Vac in Russia has shown high efficacy, immunogenicity, and a good tolerability profile in participants aged 18 years or older."
Read more: Door-to-door testing to root out 'every single case' of South Africa Covid variant
The results are calculated by how many Covid-19 cases were confirmed in both the vaccine and placebo groups from 21 days after receiving the first dose.
16 cases of symptomatic Covid-19 were confirmed in the vaccine group, compared 62 cases in the placebo group - equivalent to an efficacy of 91.6%.
Six of the 342 participants did not mount an immune response following vaccination but the study suggests this is possibly due to older age or individual characteristics.

The authors of the study note that research is needed to understand the efficacy of the vaccine on asymptomatic Covid-19 and transmission.
Four deaths were recorded during the trial - three in the vaccine group and one in the placebo group.
In the vaccine group, one death was associated with a fracture, and two had underlying conditions and developed symptoms of the virus four to five days after the first dose of the vaccine.
Both participants were deemed to have already been infected before inclusion in the trial.
In the placebo group, the death was associated with a stroke and the authors affirmed that none of the deaths were associated with jab.
Care Home CEO says some staff do not want vaccine
It has also been suggested that a combined two-stage dosage of the Sputnik vaccine and the Oxford-AstraZeneca jab could help fight against Covid-19 mutations.
The Russian Direct Investment Fund (RDIF) - the body which helped fund Russia's vaccine - said combining the two "may actually work better because immunity gets stronger".
"This idea called heterogeneous boosting," he added, "is at the core of the Sputnik vaccine because we use two different shots and we believe this is the best way to fight with mutations and this also fosters a partnership between different vaccine manufacturers."