
Iain Dale 10am - 1pm
29 January 2021, 09:10
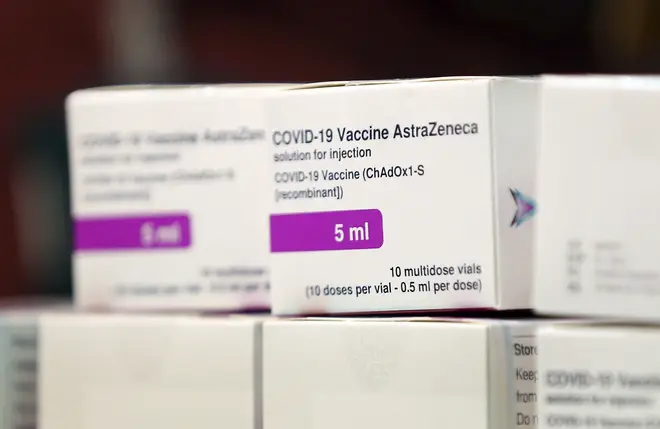
The European Union is set to approve the Oxford-AstraZeneca vaccine just 24 hours after German authorities advised over-65s against taking it.
If approved by the European Medical Agency (EMA) it will boost the EU's supplies, with hopes they will get access to millions of UK-manufactured doses.
The drug uses weakened versions of adenoviruses - a group of viruses that typically infect membranes of the eyes, respiratory tract, urinary tract, intestines and nervous system, and include the common cold.
Read more: Novavax Covid vaccine proves 89% effective in trials - with 60 million doses on order
It is not yet clear whether the regulator will set an age limit on the drug after German officials on Wednesday told its elderly population it should not receive the jab and opt for an alternative.
Top virologist reacts to Germans advising against Oxford jab
German authorities have said there is currently "not sufficient data to assess the vaccination effectiveness from 65 years".
But Public Health England (PHE) said data on the immune response for those aged 65 and over had been "reassuring".
The Prime Minister also argued that the evidence shows that the vaccine "provides a good immune response across all age groups".
Read more: German authorities advise against over-65s getting AstraZeneca Covid-19 vaccine
Deputy chair of the Joint Committee on Vaccination and Immunisation (JCVI), Professor Anthony Harnden, said Germany had far more Pfizer vaccine than Oxford/AstraZeneca supplies so wanted to prioritise its larger supply for its elderly population.
But he stressed that the Oxford jab was "absolutely safe and effective" and that the JCVI was "completely confident of this".
Race Equality Foundation chief responds to jab hesitation study
The decision comes amid a deepening row between the EU, AstraZeneca and the UK over vaccine supply shortages in the bloc.
Brussels has demanded doses be sent from British plants to make up for a shortfall, but Cabinet Office minister Michael Gove said the Government will not allow vaccines intended for the UK to go to the EU.
The President of the European Council on Wednesday night told the bloc to consider legal action to secure the doses if needed.
Read more: EU told to consider legal action amid bitter row over AstraZeneca vaccine supply
In a letter, Charles Michel said the group needs to take "robust action to secure its supply of vaccines and demonstrate concretely that the protection of our citizens take absolute priority."
Prime minister Boris Johnson has already ordered 100 million doses of the drug for the UK.
The jab is already being administered at speed throughout the UK after getting the green light from the Medicines and Healthcare products Regulatory Agency (MHRA) on December 30.