
Vanessa Feltz 3pm - 6pm
7 November 2023, 05:49 | Updated: 7 November 2023, 06:04
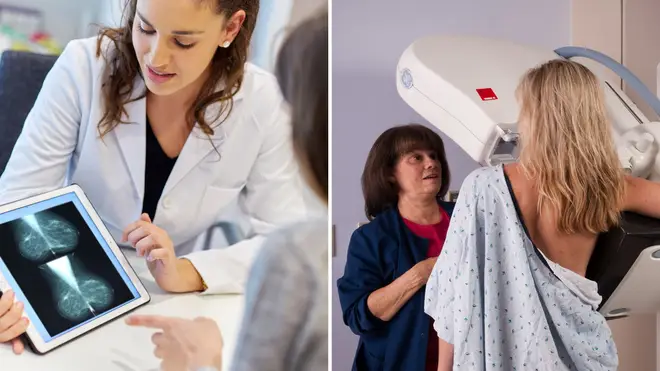
A daily pill that could cut the risk of developing breast cancer by half is to be offered to 300,000 at-risk women.
The preventative pill is set to be rolled out on the NHS to help women live “free of breast cancer”.
Studies have shown that anastrozole, which is ordinarily used as a treatment for women suffering from breast cancer, can cut the probability of developing the cancer by 49 per cent if taken daily for five years.
The drug will be offered to nearly 300,000 women in England who are considered at moderate or high risk of the disease.
If a quarter of eligible women take the drug, officials estimate it could prevent 2,000 cases in women now aged between 50 and 69.- saving the NHS roughly £15 million in treatment costs.
The drug is also cheap, costing around four pence a day.
Campaigners have hailed the approval of the drug a “major step forward” in the battle against Britain’s most common cancer, which sees around 47,000 new diagnoses each year.
Eligible women will include those who have a family history of the disease, or faulty genes such as BRCAI, which increases the risk of developing it.

Anastrozole works by cutting down the amount of oestrogen a patient’s body produces by blocking an enzyme called aromatase.
“The extension of anastrozole’s licence to cover it being used as a risk-reducing treatment is a major step forward that will enable more eligible women with a significant family history of breast cancer, to reduce their chance of developing the disease,” Baroness Morgan of Drefelin, chief executive of the charity Breast Cancer Now, said.
The drug is off-patent so it can be reproduced by multiple companies.
Regulators Medicines and Healthcare Products Regulatory Agency (MHRA) have approved the drug, making it the first medicine to be repurposed through an NHS programme.
“It's fantastic that this vital risk-reducing option could now help thousands of women and their families avoid the distress of a breast cancer diagnosis,” the head of the NHS, Amanda Pritchard, said.
“Allowing more women to live healthier lives, free of breast cancer is truly remarkable, and we hope that licensing anastrozole for a new use today represents the first step to ensuring this risk-reducing option can be accessed by all who could benefit from it.
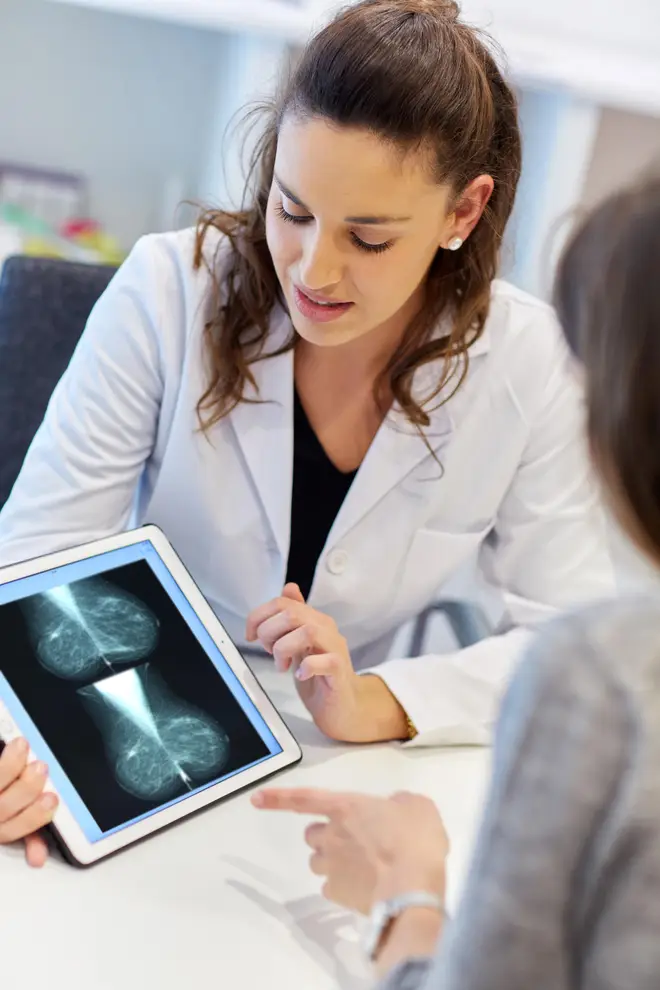
“This is the first drug to be repurposed through a world-leading new programme to help us realise the full potential of existing medicines in new uses to save and improve more lives on the NHS.
“Thanks to this initiative, we hope that greater access to anastrozole could enable more women to take risk-reducing steps if they'd like to, helping them live without fear of breast cancer.”
The medication is the first to be authorised through an NHS “repurposing” programme which is designed to see if existing medications can also be used to treat or prevent conditions.
Health Minister Will Quince said: “Breast cancer is the most common cancer in the UK so I'm delighted that another effective drug to help to prevent this cruel disease has now been approved.
“We've already seen the positive effect Anastrozole can have in treating the disease when it has been detected in post-menopausal women and now we can use it to stop it developing at all in some women.
“This is a great example of NHS England's innovative Medicines Repurposing Programme supporting the development of new ways for NHS patients to benefit from existing treatments.”