
Paul Brand 3pm - 6pm
9 November 2020, 17:04
Pfizer and BioNTech published interim results showing their jab can prevent coronavirus.
A major breakthrough has been announced in the search for a coronavirus vaccine, with the jab from Pfizer found to be more than 90% effective.
The pharmaceutical giant and its partner BioNTech said interim results showed their jab could prevent people developing Covid-19.
Dr Albert Bourla, Pfizer chairman and chief executive, said: “Today is a great day for science and humanity.”
Peter Horby, professor of emerging infectious diseases and global health at the University of Oxford, said: “This news made me smile from ear to ear.”
England’s chief medical officer, Professor Chris Whitty, said the findings demonstrated “the power of science against Covid”, adding: “We must see the final safety and efficacy data, but it is very encouraging.
“It is essential we continue to suppress Covid, but it is a reason for optimism for 2021.”
The FTSE 100 jumped more than 5.5% following the news, adding £82 billion to the value of its shares in the market’s best day since March.
The vaccine has been tested on 43,500 people in six countries and no safety concerns have been raised.
Downing Street welcomed the results as “promising” and said the UK will have procured 10 million doses by the end of the year to be given out if it is approved.
The UK has secured 40 million doses in total of the vaccine.
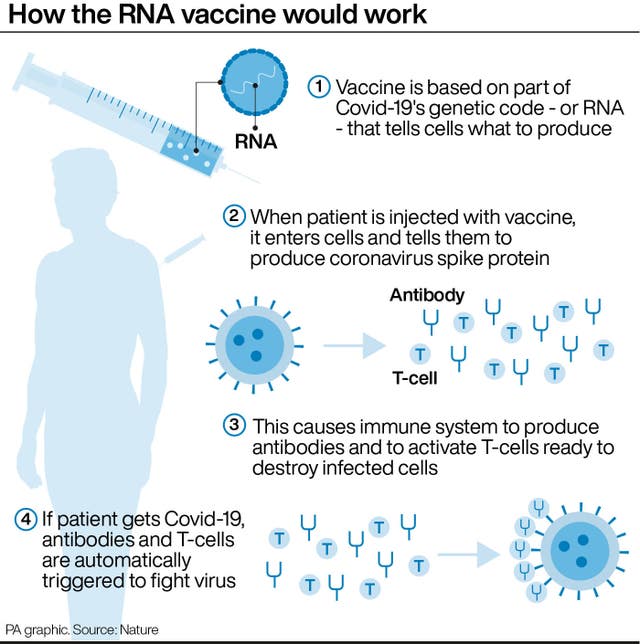
The Prime Minister’s official spokesman said: “The results are promising and while we are optimistic of a breakthrough, we must remember there are no guarantees.”
Pfizer and BioNTech plan to apply to the US Food and Drug Administration – the US medicines regulator – by the end of the month for emergency approval to use the vaccine.
About 12 Covid-19 vaccines around the world are currently in the final stages of testing, but Pfizer’s is the first to report any results.
Dr Bourla said: “The first set of results from our Phase three Covid-19 vaccine trial provides the initial evidence of our vaccine’s ability to prevent Covid-19.
“We will continue to collect further data as the trial continues to enrol, for a final analysis planned when a total of 164 confirmed Covid-19 cases have accrued.
“I would like to thank everyone who has contributed to make this important achievement possible.”

Prof Horby said the news “bodes well for Covid-19 vaccines in general”.
He added: “Of course we need to see more detail and await the final results, and there is a long long way to go before vaccines will start to make a real difference, but this feels to me like a watershed moment.”
Ian Jones, professor of virology at the University of Reading, said the Pfizer trial data shows “really impressive protection and no reported adverse events”.
He said: “Of all the current vaccines currently in development, the BioNtech product always looked like the most bang-per-buck as it is entirely focused on the part of the virus that binds to the human cell, the receptor binding domain.
“The questions around its use were about the ability to manufacture at scale and the possible toxicity associated with a directly injected RNA product.
“The trial data show excellent results in both of those areas, really impressive protection and no reported adverse events.”

David Nabarro, co-director of Imperial College London’s Institute of Global Health Innovation, said “any promising news about a vaccine is great news”. However, he cautioned that people still needed to follow rules on social distancing and wearing masks.
He told the BBC: “Even if a vaccine arrives in the near future, we’ve got many months of still dealing with the virus as a constant threat… We’ve got to make certain that we continue to do all that is necessary to solve the virus causing major problems.
“The vaccine will help, but it’s not going to be a complete game changer.”
Dr Michael Head, senior research fellow in global health, University of Southampton, added: “This cautiously sounds like an excellent result from the phase three trials, but we should remain a little cautious.
“If the final results show an effectiveness of anywhere near 90% with response in elderly and ethnic minority populations, that is an excellent result for a first generation vaccine.”
Professor Azra Ghani, chair of infectious disease epidemiology at Imperial College London, said that long-term efficacy data would come over coming weeks and months.
Prof Ghani said: “These new results represent the first demonstration of substantial efficacy of a vaccine candidate against Covid-19 disease, which is very welcome news.
“The efficacy estimate is based on seven days of follow-up of participants following the second dose; further data in the coming weeks and months will provide a better picture of longer-term vaccine efficacy.”
Preliminary news that the Pfizer/BioNTech vaccine is effective demonstrates the power of science against COVID. We must see the final safety and efficacy data, but it is very encouraging.
It is essential we continue to suppress COVID, but it is a reason for optimism for 2021.
— Professor Chris Whitty (@CMO_England) November 9, 2020
People will need two doses of the jab, meaning not enough shots have been secured for the entire UK population.
Pfizer and BioNTech expect to be able to produce up to 50 million vaccine doses globally in 2020 and up to 1.3 billion doses in 2021.
The data from the full trial will be submitted for scientific peer-review publication.
The figures presented so far are based on the first 94 volunteers to develop Covid-19.
The overall effectiveness of the vaccine may change when the full results are released.
The vaccine has been shown to produce both an antibody and T-cell response in the body to fight coronavirus.

Prof Ugur Sahin, one of the founders of BioNTech, described the results as a “milestone”.
But some experts urged caution, particularly regarding its effectiveness on the elderly.
Eleanor Riley, professor of immunology and infectious disease at the University of Edinburgh, said: “At face value, this is exceptionally good news: a vaccine that is 90% effective at preventing symptomatic cases of Covid-19 and with millions of doses available by the end of the year.
“However, the full data set on which the claim is based has not yet been released and so we don’t know exactly what has been found.
“The two companies are at pains to point out that the trial participants are ethnically diverse, which is good, but say nothing about the age of people in the trial. If a vaccine is to reduce severe disease and death, and thus enable the population at large to return to their normal day-to-day lives, it will need to be effective in older and elderly members of our society.
“We also know nothing yet about the severity of cases that were seen in the trial, whether infection or infectiousness was prevented, or how long the immunity is expected to last.
“But I think we have reason to be cautiously optimistic.”